Abstract
Introduction
Gene therapies, defined as the introduction, removal, or change in genetic material into a patient's cells to treat a specific disease, represent a significant advance in medicine, with the potential to cure or significantly improve outcomes of various benign and malignant hematologic disorders (American Society of Gene Therapy). Gene therapies have received approvals for subsets of patients with spinal muscular atrophy or retinal dystrophy, and almost a thousand studies are actively recruiting patients for various gene therapy trials. Aside from CAR-T therapies, several gene therapies are in advanced clinical development in the U.S. for disorders such as hemophilia, hemoglobinopathies, and congenital immunodeficiency syndromes. Results of many such trials were presented at the 2020 ASH annual meeting. While approvals for some gene therapy products are expected in the near future, the complexity of treatment, knowledge gaps among providers, or barriers with accessibility or cost may limit the integration of these treatments into routine clinical practice. Therefore, the present study surveyed U.S.-based community oncologists/hematologists (cO/H) to evaluate their perceptions of the utility of gene therapies and barriers to adoption or integration into clinical practice.
Methods
Between February and April 2021, cO/H from across the U.S. were invited to complete a web-based survey about gene therapies. Physician demographics and practice characteristics were also captured in the survey. Responses were aggregated and analyzed using descriptive statistics.
Results
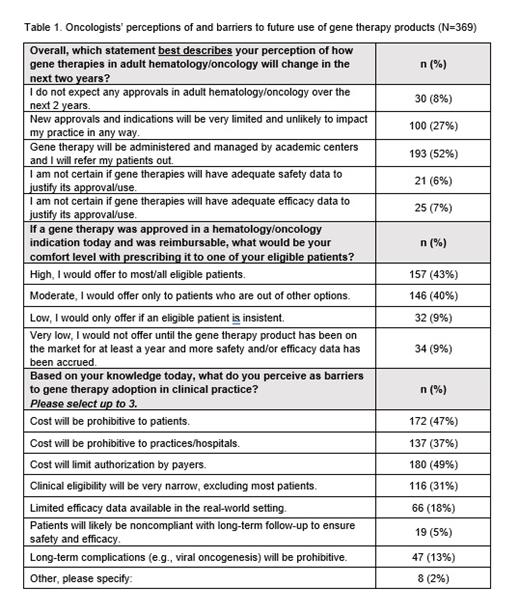
A total of 369 cO/H completed the survey; 36% identified as a medical oncologist and 63% as a hematologist/oncologist. cO/H had an average of 19 years of clinical experience and spent an average of 86% of their working time in direct patient care and saw an average of 17 patients per day on clinic days. Half of cO/H stated that they were not aware of recent data of gene therapies for adult hematology/oncology indications (35% "not very aware"; 15% "not at all aware"). When asked to report the number of approved gene therapy products (excluding CAR-T products) in the U.S. in early 2021, 27% of participants reported 0, 24% reported 1, and 20% reported 2 products were currently available. Regarding gene therapy use for adult hematologic/oncologic indications in the next 2 years, 53% of cO/H reported that they expect gene therapies to mostly be administered and managed by academic centers to which they will refer their patients; 27% reported that indications will be limited and unlikely to affect their practice (Table 1). cO/H perceived cost as the greatest barrier to adopting gene therapies into their clinical practice; specifically, cO/H cited cost limitations by payers (49%), the prohibitive cost to patients (46%), and the prohibitive cost to practices/hospitals (37%). Other barriers to adoption were limited real-world efficacy data (18%) and long-term complications (13%). Most cO/H reported a moderate (39%) or high (43%) comfort level with prescribing a gene therapy for adult hematology/oncology indications if it were reimbursed.
Conclusions
Many cO/H were not aware of recent data of gene therapy products, with over half expecting to refer their patients to large academic centers for gene therapies. While most cO/H would be comfortable prescribing gene therapies for their patients, cost was perceived as prohibitive. This information can inform various stakeholders, including patients, advocacy groups, pharmaceutical manufacturers, payers, and professional societies, in laying the foundations for gene therapy products in hematology. Future work should focus on enhancing education of gene therapy products to community providers as well as identifying support programs that lessen the burden of cost.
Gajra: Cardinal Health: Current Employment, Current equity holder in publicly-traded company. Fortier: Cardinal Health: Current Employment. Jeune-Smith: Cardinal Health: Current Employment. Feinberg: Cardinal Health: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal